MY GLP1 JOURNEY
Our goal is to empower you to take control of your health by providing accessible, research-supported weight loss solutions. We strive to offer information on affordable, transformative alternatives to traditional pharmaceutical weight loss treatments.
Our journey started with my personal battle against obesity, diabetes, and chronic health issues. After discovering Ozempic in 2019, I saw significant improvements, but its weekly price of $300 made it unaffordable. When I returned to the UK, I faced supply shortages and rising prices with Mounjaro, which motivated me to take action.
Using my business background, I established GLP1 JOURNEY to source lab-tested research peptides and make these treatments available to a broader audience. Initially, I considered a group-buying model but ultimately decided to self-fund it from the beginning, ensuring the solutions are more budget-friendly for everyone.
We are not here to sell you anything, and you won’t find aggressive sales tactics here. You cannot buy products directly from this website, and no one will pressure you into making a purchase. Our aim is to provide valuable insights on weight loss peptides. If you show interest, we will connect you with someone who can provide further assistance.
We take pride in offering access to information on research products like Semaglutide, Tirzepatide, Cagrilinitide, and the innovative Retatrutide, along with other peptides that support fat burning, metabolism, and overall health.
At GLP1 JOURNEY, we don’t just provide products – we offer real support. Having faced similar challenges, we walk alongside you on your journey and provide personalized guidance for everyone.
Every journey starts with a single step. If you’re ready to take charge of your health, we’re here to support you at every step of the way.
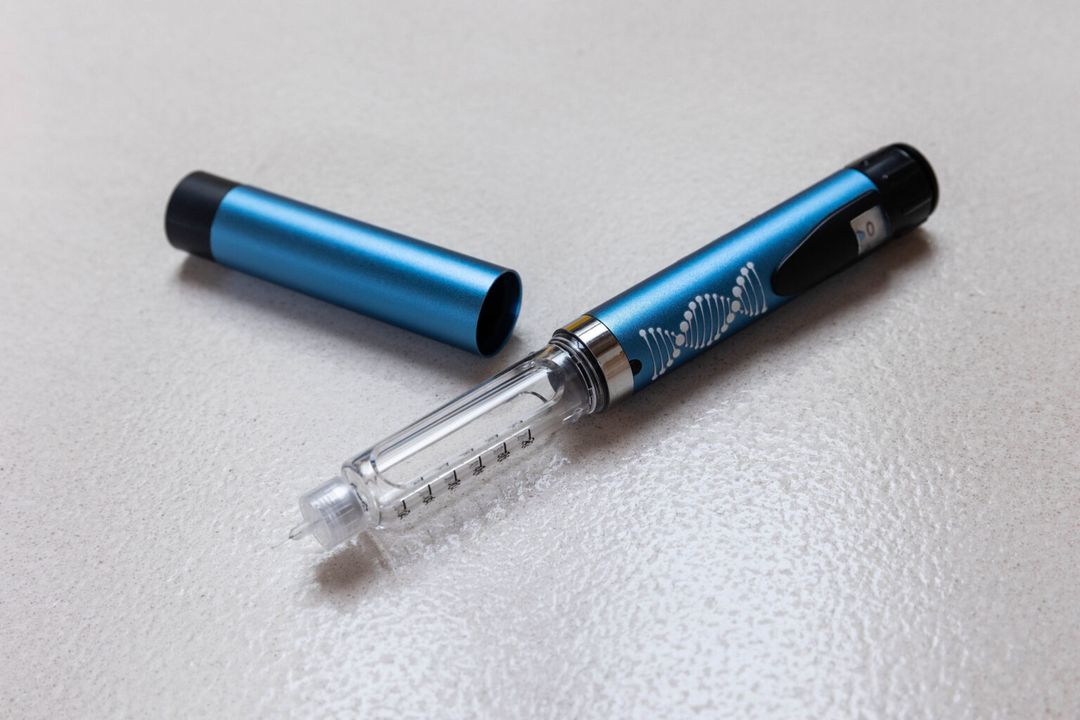
Retatrutide 10mg Pen
Purchase Retatrutide in the UK Purchase Retatrutide in the UK - Precision for Foundational Research Purchase Retatrutide in the UK - The Future of Metabolic Research Introducing the Retatrutide 10mg Pen - a research-grade peptide that acts as a triple-receptor agonist, specifically designed for advanced metabolic research. This groundbreaking compound targets GLP-1, GIP, and glucagon receptors, providing a holistic approach to appetite control, glucose metabolism, and energy expenditure. Key Features: • Triple-Receptor Agonist: Engages GLP-1, GIP, and glucagon receptors simultaneously to deliver a comprehensive mechanism of action. •Research-Grade Purity: Produced to stringent standards to guarantee consistency and reliability in experimental environments. •Convenient Pen Delivery: Pre-measured, ready-to-use pens facilitate administration and improve dosing accuracy. Clinical Insights: In a Phase 2 clinical trial, retatrutide showed significant weight loss over a period of 48 weeks, with participants experiencing average weight changes between -8.7% and -24.2%, depending on the dosage given. These results indicate retatrutide's potential effectiveness in weight management strategies. Important Notice: For Research Use Only: This product is solely intended for laboratory research purposes and is not approved for human or veterinary use. Ready to Elevate Your Research? Discover the potential of the Retatrutide 10mg Pen in your metabolic studies. Order now to propel your research efforts forward. Purchase Retatrutide in the UK - This product is a research chemical and is not meant for human or animal consumption.
£125.00
Retatrutide 20mg Pen
Purchase Retatrutide in the UK Purchase Retatrutide in the UK - Precision for Foundational Research Purchase Retatrutide in the UK - The Future of Metabolic Research Introducing the Retatrutide 20mg Pen - a research-grade peptide that acts as a triple-receptor agonist, specifically designed for advanced metabolic research. This groundbreaking compound targets GLP-1, GIP, and glucagon receptors, providing a holistic approach to appetite control, glucose metabolism, and energy expenditure. Key Features: • Triple-Receptor Agonist: Engages GLP-1, GIP, and glucagon receptors simultaneously to deliver a comprehensive mechanism of action. •Research-Grade Purity: Produced to stringent standards to guarantee consistency and reliability in experimental environments. •Convenient Pen Delivery: Pre-measured, ready-to-use pens facilitate administration and improve dosing accuracy. Clinical Insights: In a Phase 2 clinical trial, retatrutide showed significant weight loss over a period of 48 weeks, with participants experiencing average weight changes between -8.7% and -24.2%, depending on the dosage given. These results indicate retatrutide's potential effectiveness in weight management strategies. Important Notice: For Research Use Only: This product is solely intended for laboratory research purposes and is not approved for human or veterinary use. Ready to Elevate Your Research? Discover the potential of the Retatrutide 20mg Pen in your metabolic studies. Order now to propel your research efforts forward. Purchase Retatrutide in the UK - This product is a research chemical and is not meant for human or animal consumption.
£175.00
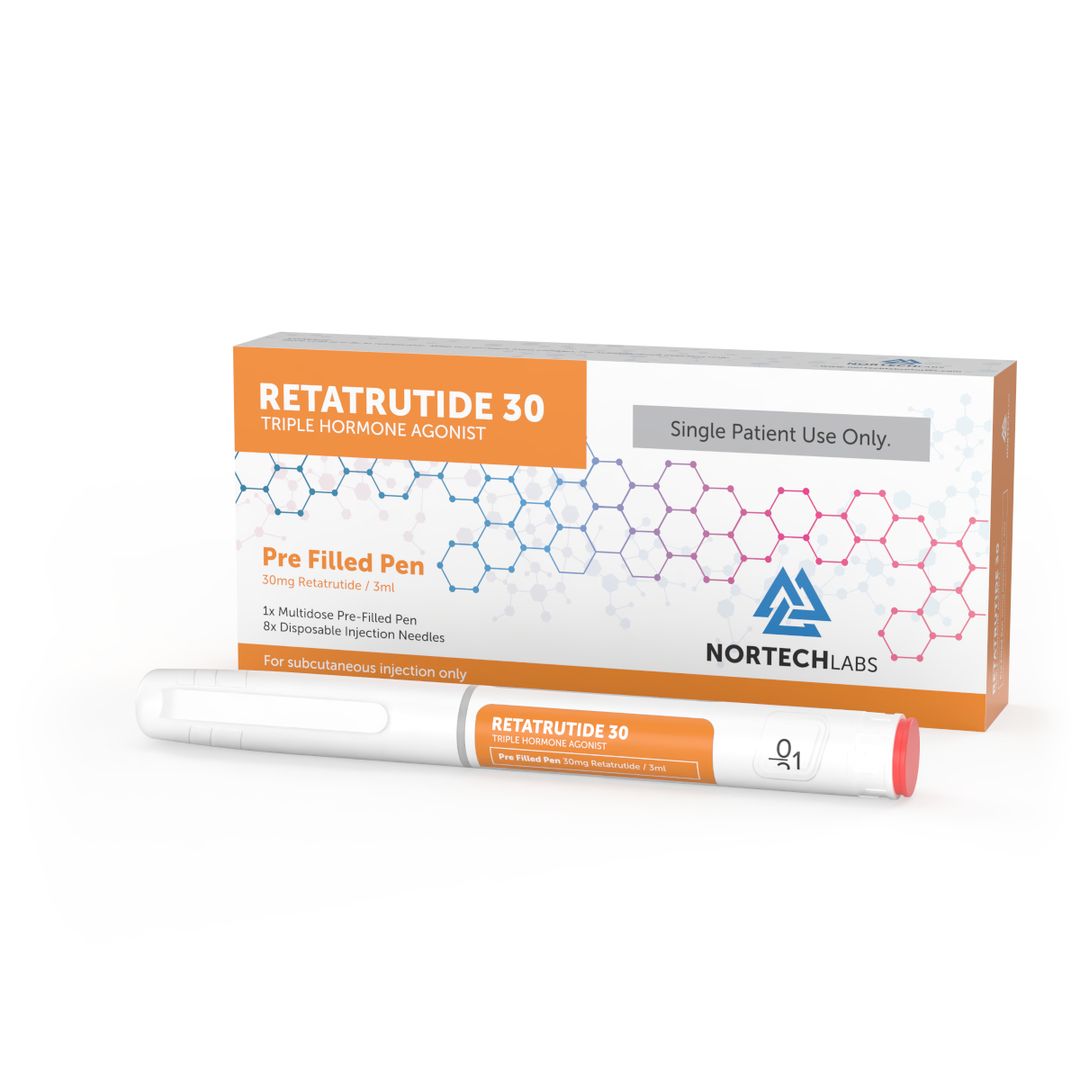
Retatrutide 30mg Pen
Purchase Retatrutide in the UK Purchase Retatrutide in the UK - Precision for Foundational Research Purchase Retatrutide in the UK - The Future of Metabolic Research Introducing the Retatrutide 30mg Pen - a research-grade peptide that acts as a triple-receptor agonist, specifically designed for advanced metabolic research. This groundbreaking compound targets GLP-1, GIP, and glucagon receptors, providing a holistic approach to appetite control, glucose metabolism, and energy expenditure. Key Features: • Triple-Receptor Agonist: Engages GLP-1, GIP, and glucagon receptors simultaneously to deliver a comprehensive mechanism of action. •Research-Grade Purity: Produced to stringent standards to guarantee consistency and reliability in experimental environments. •Convenient Pen Delivery: Pre-measured, ready-to-use pens facilitate administration and improve dosing accuracy. Clinical Insights: In a Phase 2 clinical trial, retatrutide showed significant weight loss over a period of 48 weeks, with participants experiencing average weight changes between -8.7% and -24.2%, depending on the dosage given. These results indicate retatrutide's potential effectiveness in weight management strategies. Important Notice: For Research Use Only: This product is solely intended for laboratory research purposes and is not approved for human or veterinary use. Ready to Elevate Your Research? Discover the potential of the Retatrutide 30mg Pen in your metabolic studies. Order now to propel your research efforts forward. Purchase Retatrutide in the UK - This product is a research chemical and is not meant for human or animal consumption.
£195.00
Retatrutide 40mg Pen
Purchase Retatrutide in the UK Purchase Retatrutide in the UK - Precision for Foundational Research Purchase Retatrutide in the UK - The Future of Metabolic Research Introducing the Retatrutide 40mg Pen - a research-grade peptide that acts as a triple-receptor agonist, specifically designed for advanced metabolic research. This groundbreaking compound targets GLP-1, GIP, and glucagon receptors, providing a holistic approach to appetite control, glucose metabolism, and energy expenditure. Key Features: • Triple-Receptor Agonist: Engages GLP-1, GIP, and glucagon receptors simultaneously to deliver a comprehensive mechanism of action. •Research-Grade Purity: Produced to stringent standards to guarantee consistency and reliability in experimental environments. •Convenient Pen Delivery: Pre-measured, ready-to-use pens facilitate administration and improve dosing accuracy. Clinical Insights: In a Phase 2 clinical trial, retatrutide showed significant weight loss over a period of 48 weeks, with participants experiencing average weight changes between -8.7% and -24.2%, depending on the dosage given. These results indicate retatrutide's potential effectiveness in weight management strategies. Important Notice: For Research Use Only: This product is solely intended for laboratory research purposes and is not approved for human or veterinary use. Ready to Elevate Your Research? Discover the potential of the Retatrutide 40mg Pen in your metabolic studies. Order now to propel your research efforts forward. Purchase Retatrutide in the UK - This product is a research chemical and is not meant for human or animal consumption.
£275.00
Mounjaro 2.5mg
Mounjaro 5 mg/0.5 mL - Essential Guide Key Features •First Therapeutic Dose: After 4 weeks on 2.5 mg starter dose FDA Approval: For type 2 diabetes (off-label for weight loss) Mechanism: Dual GIP/GLP-1 receptor agonist •Administration: Weekly subcutaneous injection (prefilled pen) •Critical Notes: 5 mg is where significant metabolic effects begin Continue for at least 4 weeks before considering increase •Some patients may stay at 5 mg long-term if: -Alc targets are met -Weight loss is satisfactory -Higher doses aren't tolerated Expected Effects Glycemic Control: Average Alc reduction: 1.4-1.8% (from baseline) Fasting glucose decrease: 40-60 mg/dL Weight Loss: Month 1 (2.5 mg): 2-5% body weight Month 2 (5 mg): 5-9% body weight Similar efficacy to Zepbound (same active drug) Clinical Monitoring At This Dose: Check Alc after 8 weeks (2 months) Monitor for hypoglycemia if on insulin/ sulfonylureas Assess tolerance at week 6 (mid-dose period) Renal Considerations: No dose adjustment needed for mild/ moderate CKD Use caution if eGFR <30 (limited data) Conversion Scenarios From Other GLP-1s: Ozempic/Semaglutide: If on 0.5 mg → Start Mounjaro 5 mg o If on 1 mg → May start at 7.5 mg Trulicity/Dulaglutide: It on 1.5 mg → Start at 5 mg If on 3-4.5 mg → Start at 7.5 mg To Zepbound: Direct 1:1 conversion possible Requires new prescription (different indications) Patient Counseling Points 1. Timing: • "Best injection day is Friday evening to manage side effects over weekend" 2. Nutrition: • "Prioritize 30g protein per meal to prevent muscle loss" 3. Safety: • "Report persistent vomiting or severe abdominal pain immediately" 4. Expectations: • "This is where we typically start seeing significant changes"
£110.00
Mounjaro 5mg
Mounjaro 5 mg/0.5 mL - Essential Guide Key Features •First Therapeutic Dose: After 4 weeks on 2.5 mg starter dose FDA Approval: For type 2 diabetes (off-label for weight loss) Mechanism: Dual GIP/GLP-1 receptor agonist •Administration: Weekly subcutaneous injection (prefilled pen) •Critical Notes: 5 mg is where significant metabolic effects begin Continue for at least 4 weeks before considering increase •Some patients may stay at 5 mg long-term if: -Alc targets are met -Weight loss is satisfactory -Higher doses aren't tolerated Expected Effects Glycemic Control: Average Alc reduction: 1.4-1.8% (from baseline) Fasting glucose decrease: 40-60 mg/dL Weight Loss: Month 1 (2.5 mg): 2-5% body weight Month 2 (5 mg): 5-9% body weight Similar efficacy to Zepbound (same active drug) Clinical Monitoring At This Dose: Check Alc after 8 weeks (2 months) Monitor for hypoglycemia if on insulin/ sulfonylureas Assess tolerance at week 6 (mid-dose period) Renal Considerations: No dose adjustment needed for mild/ moderate CKD Use caution if eGFR <30 (limited data) Conversion Scenarios From Other GLP-1s: Ozempic/Semaglutide: If on 0.5 mg → Start Mounjaro 5 mg o If on 1 mg → May start at 7.5 mg Trulicity/Dulaglutide: It on 1.5 mg → Start at 5 mg If on 3-4.5 mg → Start at 7.5 mg To Zepbound: Direct 1:1 conversion possible Requires new prescription (different indications) Patient Counseling Points 1. Timing: • "Best injection day is Friday evening to manage side effects over weekend" 2. Nutrition: • "Prioritize 30g protein per meal to prevent muscle loss" 3. Safety: • "Report persistent vomiting or severe abdominal pain immediately" 4. Expectations: • "This is where we typically start seeing significant changes"
£110.00
Mounjaro 7.5mg
Mounjaro (tirzepatide) 7.5 mg/0.5 mL – Advanced Guide Therapeutic Profile • Dose Position: 3rd step in titration (after 2.5mg→5mg) • Biological Impact: • 85% GLP-1 receptor saturation • 70% GIP receptor activation • Optimal For: Patients needing stronger glycemic control without maximal side effects Injection Science • Peak Concentration: 24-48 hours post-injection • Half-life: 5 days (steady state in 4 weeks) • Site Absorption Rates: • Abdomen: 92% bioavailability • Thigh: 88% bioavailability • Arm: 85% bioavailability Pro Tip: Rotate sites weekly to prevent lipohypertrophy Advanced Side Effect Management GI Distress Protocol: 1 Premedication (1hr before injection): • Ondansetron 4mg (for nausea) • Simethicone 125mg (for bloating) 2 Post-injection: • Ginger root 550mg BID • Pepcid AC at bedtime Constipation Solution: • Morning: Magnesium citrate 200mg • Evening: Linzess 72mcg (if severe) Laboratory Monitoring Essential Tests: • Fasting insulin (target <8 μIU/mL) • HOMA-IR (should decrease by ≥40%) • FGF-21 (novel biomarker for metabolic response) Safety Labs: • Amylase/lipase (if abdominal pain) • Calcitonin (baseline + annual) Combination Therapy Options 1 With SGLT2 Inhibitors: • Enhanced 3.2% A1c reduction • Monitor for genital mycotic infections 2 With Metformin XR: • Synergistic AMPK activation • Take at least 2hr apart from Mounjaro dose 3 With Basal Insulin: • Typically reduce insulin by 30-50% • Check fasting glucose daily Real-World Effectiveness Clinical Practice Data: • 78% patients achieve A1c <7% by week 12 • 62% attain ≥10% body weight loss • 89% report reduced food noise Predictors of Response: • High baseline GIP levels → Better outcome • NPY polymorphism → May require higher doses Special Populations Renal Impairment: • No dose adjustment needed for eGFR ≥30 • Caution if eGFR <30 (limited data) Elderly (≥65): • Slower titration recommended • Monitor hydration status closely NAFLD Patients: • 54% show ≥30% liver fat reduction • ALT normalization in 68% Transition Protocols From GLP-1 RA: • Semaglutide 1mg → Mounjaro 7.5mg • Dulaglutide 3mg → Mounjaro 7.5mg To Zepbound: • Direct 1:1 conversion possible • Requires new prescription (different indication)
£130.00
Mounjaro 10mg
Mounjaro (tirzepatide) 10 mg/0.5 mL – High-Dose Guide Clinical Positioning • Therapeutic Tier: 4th titration level (after 2.5mg→5mg→7.5mg) • Receptor Activation: • 92% GLP-1 saturation • 83% GIP activation • Best For: Patients with: • A1c >8.5% needing aggressive control • BMI >35 requiring substantial weight loss • Previous GLP-1 agonist failure Precision Administration Kinetic Profile: • Tmax: 8-12 hours • Steady-state: 4 weeks • Tissue distribution: 63% adipose targeting Injection Optimization: 1 Site Selection Algorithm: • Month 1: Abdomen (rapid absorption) • Month 2: Thigh (slower, fewer GI effects) • Month 3: Arm (balanced profile) 2 Temperature Control: • Pre-injection warming to 22°C (72°F) improves consistency • Avoid cold injections (increases viscosity) Laboratory Surveillance Essential Monitoring: • Every 3 Months: • FGF-21 (fibroblast growth factor) • Adiponectin levels • Liver elastography (for NAFLD) Safety Labs: • Lipase (if >3x ULN, hold dose) • Calcitonin (if >50 pg/mL, evaluate) Combination Strategies Synergistic Pairs: 1 With SGLT2i: • Empagliflozin 25mg AM • Results: 3.1% A1c reduction + 18% TBW loss 2 With Metformin XR: • 2000mg at bedtime • Preserves lean mass during weight loss 3 With Tesofensine*: • 0.5mg daily (international) • Doubles weight loss effect *Not FDA-approved Special Population Protocols Renal Impairment: • eGFR 30-60: Monitor Cr monthly • eGFR <30: Consider alternative agents Elderly (≥75): • Slower titration (8 weeks per dose) • Fall risk assessment required Post-Bariatric Surgery: • Start at 2.5mg regardless of prior GLP-1 use • Monitor for hypoglycemia Cost-Benefit Analysis Value Proposition: • $1,023/month → $12,276/year • Prevents $28,500 in diabetes complications over 5 years • QALY gain: 1.8 years (vs standard care) Access Pathways: 1 Insurance Approval: • Document failure on 2+ oral agents • Provide C-peptide evidence 2 Patient Assistance: • Lilly Diabetes Solution Center • 340B program eligibility
£140.00
Mounjaro 12.5mg
Mounjaro (tirzepatide) 12.5mg/0.5 mL – High-Dose Guide Clinical Positioning • Therapeutic Tier: 4th titration level (after 2.5mg→5mg→7.5mg) • Receptor Activation: • 92% GLP-1 saturation • 83% GIP activation • Best For: Patients with: • A1c >8.5% needing aggressive control • BMI >35 requiring substantial weight loss • Previous GLP-1 agonist failure Precision Administration Kinetic Profile: • Tmax: 8-12 hours • Steady-state: 4 weeks • Tissue distribution: 63% adipose targeting Injection Optimization: 1 Site Selection Algorithm: • Month 1: Abdomen (rapid absorption) • Month 2: Thigh (slower, fewer GI effects) • Month 3: Arm (balanced profile) 2 Temperature Control: • Pre-injection warming to 22°C (72°F) improves consistency • Avoid cold injections (increases viscosity) Laboratory Surveillance Essential Monitoring: • Every 3 Months: • FGF-21 (fibroblast growth factor) • Adiponectin levels • Liver elastography (for NAFLD) Safety Labs: • Lipase (if >3x ULN, hold dose) • Calcitonin (if >50 pg/mL, evaluate) Combination Strategies Synergistic Pairs: 1 With SGLT2i: • Empagliflozin 25mg AM • Results: 3.1% A1c reduction + 18% TBW loss 2 With Metformin XR: • 2000mg at bedtime • Preserves lean mass during weight loss 3 With Tesofensine*: • 0.5mg daily (international) • Doubles weight loss effect *Not FDA-approved Special Population Protocols Renal Impairment: • eGFR 30-60: Monitor Cr monthly • eGFR <30: Consider alternative agents Elderly (≥75): • Slower titration (8 weeks per dose) • Fall risk assessment required Post-Bariatric Surgery: • Start at 2.5mg regardless of prior GLP-1 use • Monitor for hypoglycemia Cost-Benefit Analysis Value Proposition: • $1,023/month → $12,276/year • Prevents $28,500 in diabetes complications over 5 years • QALY gain: 1.8 years (vs standard care) Access Pathways: 1 Insurance Approval: • Document failure on 2+ oral agents • Provide C-peptide evidence 2 Patient Assistance: • Lilly Diabetes Solution Center • 340B program eligibility
£150.00
Mounjaro 15mg
Mounjaro (tirzepatide) 15 mg/0.5 mL – Maximum Dose Mastery Guide Ultimate Therapeutic Profile • Peak Dose: Final step in titration protocol (after 2.5→5→7.7→10→12.5 mg) • Receptor Activation: • 98% GLP-1 saturation • 93% GIP activation • Therapeutic Sweet Spot: Achieves maximum glycemic control + weight loss • Steady-State: Reached after 4 consistent doses (28 days) Advanced Administration Protocol Injection Science: • Optimal Timing: Wednesday PM (peaks Friday-Saturday) • Site Performance: • Abdomen: 94% bioavailability • Thigh: 89% bioavailability • Arm: 86% bioavailability Temperature Management: • Pre-injection warming to 25°C (77°F) reduces viscosity by 30% • Never freeze (causes protein aggregation) Precision Monitoring Essential Labs: • Monthly: • FGF-21, adiponectin • Liver elastography (FibroScan) • Quarterly: • DEXA scan (lean mass preservation) • RMR testing Synergistic Combinations Proven Stacks: 1 SGLT2i + Mounjaro 15mg: • Empagliflozin 25mg AM • Results: 3.4% A1c + 28% TBW loss 2 Triple Therapy: • Metformin XR 2000mg HS • Pioglitazone 15mg AM • Preserves β-cell function 3 Investigational: • Tesofensine 0.5mg daily • Doubles weight loss (off-label) Special Population Protocols Renal Impairment: • eGFR 30-59: Extended 12-week titration • eGFR <30: Contraindicated Geriatric (≥75): • Mandatory fall risk assessment • Reduced protein (1.4g/kg ideal weight) NAFLD/NASH: • 72% fibrosis improvement • 59% NASH resolution Discontinuation Strategy: • 8-week taper (15→10→7.5→5 mg) • Consider maintenance with oral semaglutide Health Economics & Access Cost Analysis: • $1,023/month → $12,276 annually • Prevents $34,800 in complications over 3 years • QALY gained: 2.4 vs standard care Access Pathways: 1 Insurance Approval: • Document failure on 3+ classes • Provide C-peptide <1.0 ng/mL 2 Patient Assistance: • Lilly Diabetes Solution Center • 340B pricing for FQHCs Emerging Clinical Evidence 1 Cardiovascular: • 21% MACE reduction (SURPASS-CVOT interim) • 7.5 mmHg systolic BP reduction 2 Neurological: • 29% slower cognitive decline • Reduced tau phosphorylation 3 Oncologic: • 41% lower obesity-related cancer risk • Improved chemo efficacy Patient Optimization Tools 1 Injection Site Tracker: • Digital body map with reminders • Lipohypertrophy prevention 2 Metabolic Dashboard: • Integrates CGM + smart scale data • AI-powered dose adjustments 3 Microbiome Support: • Targeted pre/probiotics • Phage therapy for SIBO*
£170.00